NEXPLANON®
(etonogestrel implant) 68 mg radiopaque

NEXPLANON®
(etonogestrel implant) 68 mg radiopaque


(etonogestrel implant) 68 mg radiopaque
All HCPs performing insertions and/or removals of NEXPLANON should receive instructions and training prior to inserting or removing the implant.
If you require certification to administer NEXPLANON, you’ll find all the resources you need here to register and request training. If you are already registered and trained, you can access your certification document and review training materials.
aPlaced subdermally just under the skin in the inner, non-dominant upper arm.
Learn more about the insertion of NEXPLANON
The video below is for informational purposes only and is not intended to serve as a substitute for training on the insertion and removal procedures for NEXPLANON.
Download instructions for inserting and removing NEXPLANON
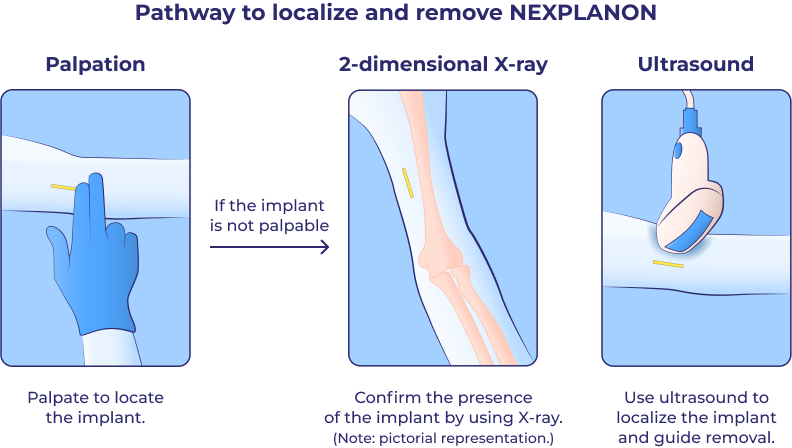
Palpation is an important technique for locating NEXPLANON after insertion and before removal.
The NEXPLANON implant is radiopaque, providing 4 methods for confirming presence after insertion and localizing before removal, in the event that the implant is not palpable.
CT = computed tomography; MRI = magnetic resonance imaging.
![]()
Study Design1
In a clinical trial evaluating the insertion characteristics of the applicator for NEXPLANON:
Out of 301 insertions of the NEXPLANON implant, the mean insertion timeb was (±SD) 27.9±29.3 seconds.
bFrom the removal of the protective cap of the applicator until retraction of the needle from the arm.
SD = standard deviation.
If inserted as recommended, backup contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
Timing of insertion depends on the woman’s recent contraceptive history, as follows:
If switching from combination hormonal contraception:
If switching from progestin-only contraception:
Following abortion or miscarriage
Postpartum
Breastfeeding: NEXPLANON should not be inserted until after the fourth postpartum week. The woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
IUS = intrauterine system.
HCP = healthcare professional.
NEXPLANON is indicated for use by women to prevent pregnancy.
Complications of Insertion and Removal
Broken or Bent Implants
Changes in Menstrual Bleeding Patterns
Ectopic Pregnancies
Thrombotic and Other Vascular Events
Ovarian Cysts
Carcinoma of the Breast and Reproductive Organs
Liver Disease
Elevated Blood Pressure
Gallbladder Disease
Carbohydrate and Lipid Metabolic Effects
Depressed Mood
Return to Ovulation
Fluid Retention
Contact Lenses
Clinical Trial Experience
Effects of Other Drugs on Hormonal Contraceptives
Substances decreasing the plasma concentrations of hormonal contraceptives and potentially diminishing the efficacy of hormonal contraceptives:
Substances increasing the plasma concentrations of hormonal contraceptives:
Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors:
Effects of Hormonal Contraceptives on Other Drugs
USE IN SPECIFIC POPULATIONS
Pregnancy
Lactation
Pediatric Use
Overweight Women
Before prescribing NEXPLANON, please read the accompanying Prescribing Information. The Patient Information also is available.
1. Mansour D, Mommers E, Teede H, et al. Clinician satisfaction and insertion characteristics of a new applicator to insert radiopaque Implanon: an open-label, noncontrolled, multicenter trial. Contraception. 2010;82(3):243–249.